HOME
ELEMENTS
Crystal
Crystal is defined as a homogeneous (uniform or the same throughout)
portion of matter that has a definite, orderly atomic structure, and
an outward form bounded by smooth, plane surfaces, symmetrically
arranged, and is produced whenever a solid is formed gradually from a
fluid, whether the formation results from the  freezing
of a liquid, the deposition of dissolved matter, or the direct
condensation of a gas into solid form. The angles between
corresponding faces of any two crystals of the same substance,
regardless of size or superficial difference of form, are always
identical. Most solid matter displays orderly atomic arrangement and
is of crystalline structure. Solids that have no crystalline
structure, such as glass, are called amorphous. In structure they
show greater similarity to liquids than to solids, and are known as
supercooled liquids.
freezing
of a liquid, the deposition of dissolved matter, or the direct
condensation of a gas into solid form. The angles between
corresponding faces of any two crystals of the same substance,
regardless of size or superficial difference of form, are always
identical. Most solid matter displays orderly atomic arrangement and
is of crystalline structure. Solids that have no crystalline
structure, such as glass, are called amorphous. In structure they
show greater similarity to liquids than to solids, and are known as
supercooled liquids.
If a solution
remains undisturbed as it is cooled, it often passes the point at
which it would normally crystallize, reaching a supercooled state.
The same is true for a solution containing a maximum amount of
solute; if it assimilates additional solute, it is termed
supersaturated. In both instances, if a tiny crystal, called a seed
crystal, is added to the solution, a sudden chain reaction occurs, as
shown here, and crystal growth is dramatic.
Cooling rate
The same liquids that gradually freeze deep within the earth to form granite
are sometimes ejected at the surface as volcanic lava and cool
quickly, forming a glassy rock called obsidian. If the cooling
is slightly slower, a rock called felsite is formed; it is
crystalline, but the crystals are too small to be seen with the naked
eye. Such a structure is called cryptocrystalline, or aphanitic.
Still slower cooling results in a rock of porphyritic structure, in
which some of the crystals are large enough to be visible; this rock,
which may be of identical composition with obsidian, felsite, or
granite, is called rhyolite. All of these minerals are the
same atomic structure.
Crystal growth
Crystal growth is attained when a microscopic crystal that has formed
condences more of the same element from its environment. Sometimes,
in the absence of this first minute crystal, or seed, crystallization
does not take place, and the solution becomes supersaturated (more
highly concentrated than is normally possible under given temperature
and pressure), just as a liquid below its freezing point becomes
supercooled. When a new organic chemical is prepared, it is often
difficult to make the first crystal unless a substance with simular
form can be found. The tendency to crystallize decreases with
increasing viscosity (high resistance to flow) of the liquid; if a
solution becomes considerably supersaturated or supercooled it
becomes very viscous, and crystallization becomes almost impossible.
Further cooling or evaporation of the solvent produces first a syrup
and then a glass.
Some substances have a strong tendency to form seed crystals. If a
solution of such a substance is cooled slowly, a few seeds grow into
large crystals; but if it is cooled rapidly, numerous seeds form and
grow only into tiny crystals. Table salt, purified at a factory by
recrystallization, is composed of numerous perfect cubic crystals,
which are barely visible to the naked eye; rock salt, formed by the
slow processes of geology, contains enormous crystals of the same
cubic form.
Crystallography
The study of the growth, shape, and geometric character of crystals
is called crystallography. When conditions are favorable, each
chemical element and compound tends to crystallize in a definite and
characteristic form. Thus, salt tends to form cubic crystals; but
garnet, which also occasionally forms cubes, more commonly occurs in
dodecahedrons (solids with 12 faces) or trisoctahedrons (solids with
24 faces). Despite their differences in habit (shape of
crystallization), salt and garnet always crystallize in the same
class and system. Thirty-two classes of crystal are theoretically
possible; almost all common minerals fall into one of about twelve
classes, and some classes have never been observed. The thirty-two
classes are grouped into six crystal systems, based on the length and
position of the crystal axes, imaginary lines passing through the
center of the crystal, intersecting the faces, and bearing definite
relations to the symmetry of the crystal. Minerals in each system
share certain details of symmetry and crystal form and many important
optical properties.
The Six Crystal Systems
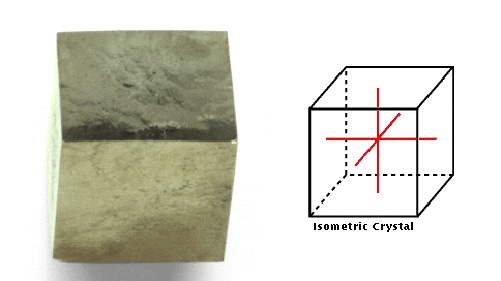 Isometric
Isometric
This system comprises crystals with three axes, all perpendicular to
one another and all equal in length.
Isometric
crystals, such as the pyrite shown here, have three perpendicular
axes of equal length. The cubic-isometric structure is the most
symmetrical of all the crystals. The pyrite crystal system forms
rocks that are fairly hard, but quite brittle. Pyrite is also known
as fool's gold because of its yellow color and metallic luster.
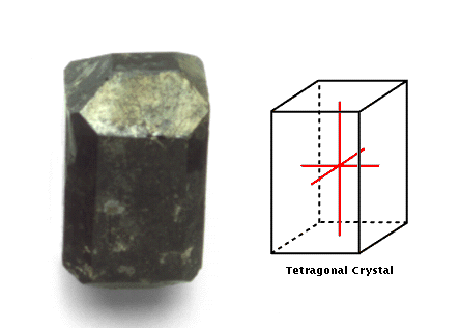 Tetragonal
Tetragonal
This system comprises crystals with three axes, all perpendicular to
one another; two are of equal length.
This Siberian
idocrase has a tetragonal crystal structure. Its axes are all
perpendicular and two are of equal length. Idocrase is grouped with
rocks such as zircon, rutile, and wulfenite, which are rocks of
medium hardness that may possess a diamond-like fire.
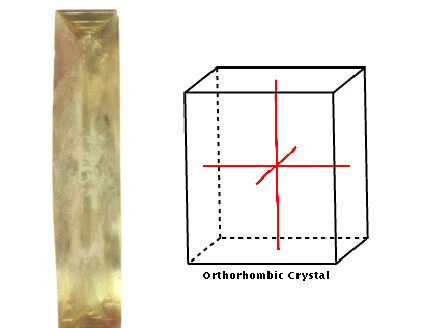 Orthorhombic
Orthorhombic
This system comprises crystals with three mutually perpendicular
axes, all of different lengths.
Barite, from
which barium comes, has an orthorhombic crystal structure. It has
three mutually perpendicular axes of different lengths. Barite
exhibits perfect cleavage, which means it splits easily along
specific, intersecting planes.
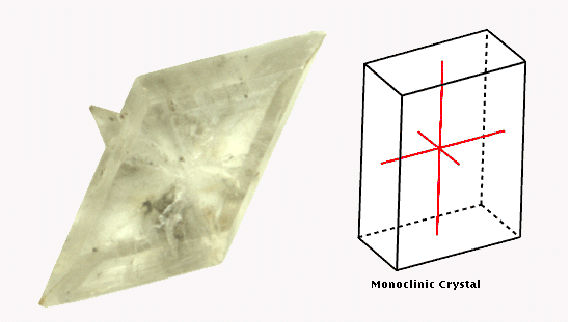 Monoclinic
Monoclinic
This system comprises crystals with three axes of unequal lengths,
two of which are oblique (not perpendicular) to one another, but both
of which are perpendicular to the third.
Gypsum is an
example of a mineral exhibiting a monoclinic crystal structure.
Monoclinic crystals have three axes of unequal length, two of which
are perpendicular to the third axis, but not to each other. A soft,
sedimentary rock, gypsum is the source of plaster of Paris and also
has applications in agriculture and construction.
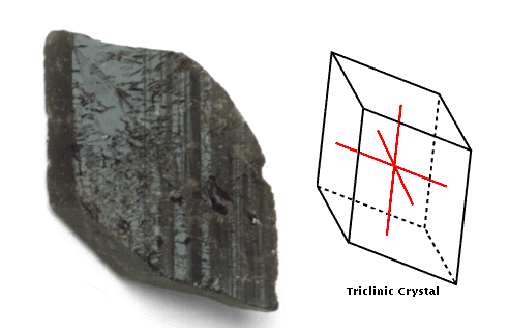 Triclinic
Triclinic
This system comprises crystals with three axes, all unequal in length
and oblique to one another.
Triclinic
crystals exhibit the least symmetry of the crystal systems. Their
axes are unequal and do not intersect at right angles anywhere. This
Brazilian axinite is an example of a triclinic crystal.
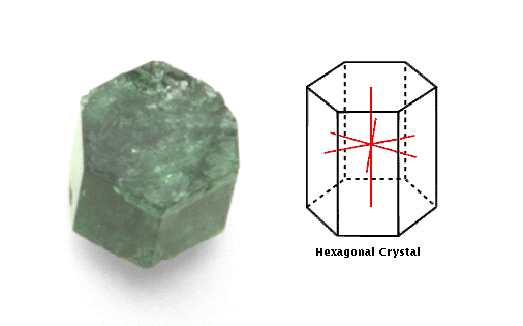 Hexagonal
Hexagonal
This system comprises crystals with four axes. Three of these axes
are in a single plane, symmetrically spaced, and of equal length. The
fourth axis is perpendicular to the other three
A hexagonal
crystal such as beryl, shown here, has four axes of symmetry. Three
of the axes are of equal length, and are symmetrically placed in one
plane. The fourth axis is perpendicular to the others.
Some crystallographers split the hexagonal
system in two, calling the seventh system thus formed trigonal or rhombohedral.
A few elements and compounds can crystallize in two
different systems, giving rise to substances which, although
identical in chemical composition, are different in virtually all
their physical properties. For example, carbon crystallizes in the
isometric system to form diamond, and in the hexagonal system to form
graphite. Although diamond is in the same system as salt and garnet,
it is in a different class. It crystallizes in tetrahedrons (solids
with 4 faces) or octahedrons (solids with 8 faces); the latter is
possible in the garnet-salt class, the former is not.
Other Crystal Properties
The habit of any mineral includes many other
properties based on crystalline structure. For example, argentite, a
common silver ore, crystallizes in the same class as garnet and salt,
but usually occurs in irregular cryptocrystalline masses. Fluorite,
another common mineral, also crystallizes in the same class and
usually forms cubes; when fractured, florite tends to cleave into
perfect octahedral fragments. Salt forms cubic cleavage fragments,
and garnet has no well-developed cleavage planes. Some substances
tend to form multiple crystals growing through one another.
Some crystals, when compressed, develop electrical
charges at their ends; other crystals develop similar charges when
heated. These properties, called piezoelectricity and pyroelectricity
respectively, are both shown to a marked degree by quartz. For this
reason, quartz crystals are used in sonar and in many types of radio
apparatus. In the transistor special properties of germanium and
silicon crystals are utilized for amplifying electric current.
Another electronic device, the solar battery, utilizes a silicon or
cadmium sulfide crystal to convert sunlight into electrical energy.
Much work has been done in recent years on the
preparation of single crystals of substances that are normally
cryptocrystalline. Large, single crystals of metals, for example, can
be grown by a number of methods, the simplest of which is to melt the
metal in a conical vessel, and then lower the vessel slowly from the
furnace, point first. If the proper conditions exist, a single seed
forms at the point of the cone and continues to grow until it has
filled the vessel. Such single crystals often differ markedly from
metals in their usual form. Pure and specially designed crystals are
now also produced by advanced techniques such as molecular-beam
epitaxy for use in semiconducting devices, integrated circuits, and
various other important systems of modern technology.
When X rays pass through the symmetrically arranged
atoms of a crystal, the atoms act as a diffraction grating,
deflecting the rays in regular patterns. Photographs taken of these
patterns give scientists a basis for deducing many facts concerning
the nature of the crystal. The actual arrangements of atoms in
crystals can be revealed in images produced by transmission electron
microscopes (see Microscope) and field-ion emission devices.
One basic rule of crystallography has long been that
no crystal structure can have fivefold, or pentagonal, symmetry. It
was thought that such symmetry could not exhibit the translational
periodicity required of crystals. In 1984, however, a group of
scientists found an alloy of aluminum and magnesium that seems to
break that rule. The alloy's diffraction pattern shows it to have the
rotational symmetry of an icosahedron, or 20-faced solid, with 10
threefold axes and 6 fivefold axes of rotational symmetry. This
discovery opens up the possibility that a whole new phase of
organized solid matter may exist, distinct from crystals and glasses.
"Crystal (mineral)," Microsoft®
Encarta® Encyclopedia 99. © 1993-1998 Microsoft Corporation.
All rights reserved.
 freezing
of a liquid, the deposition of dissolved matter, or the direct
condensation of a gas into solid form. The angles between
corresponding faces of any two crystals of the same substance,
regardless of size or superficial difference of form, are always
identical. Most solid matter displays orderly atomic arrangement and
is of crystalline structure. Solids that have no crystalline
structure, such as glass, are called amorphous. In structure they
show greater similarity to liquids than to solids, and are known as
supercooled liquids.
freezing
of a liquid, the deposition of dissolved matter, or the direct
condensation of a gas into solid form. The angles between
corresponding faces of any two crystals of the same substance,
regardless of size or superficial difference of form, are always
identical. Most solid matter displays orderly atomic arrangement and
is of crystalline structure. Solids that have no crystalline
structure, such as glass, are called amorphous. In structure they
show greater similarity to liquids than to solids, and are known as
supercooled liquids. Isometric
Isometric Tetragonal
Tetragonal Orthorhombic
Orthorhombic Monoclinic
Monoclinic Triclinic
Triclinic Hexagonal
Hexagonal